
The most typical reaction of alkynes just like alkenes is the electrophilic addition to a π-bond. Similar to alkenes, the nomenclature of alkynes involves the change of the – ane ending of an alkane functional group with an – yne ending for the alkyne. This might not make much sense now, but we’ll get back to it later when we start talking about various aspects of the structure and bonding in organic molecules. This gives a huge amount of angular strain in the molecule making cyclic alkynes very unstable. The problem with cyclic alkynes and the reason for their rarity is the fact that the carbons of the triple bond are sp-hybridized which means they have a 180° bond angle. Nonetheless, cyclic alkynes also do exist. Most alkynes that you are going to encounter within the scope of this course are going to be an open-chain style molecules. AlkynesĪlkynes contain a carbon-carbon triple bond. So, this is one of those functional groups that we are going to be coming back to from time to time. However, we are going to see more different reactions of alkenes later in the course. The typical reaction of alkenes is the electrophilic addition to a double bond. Additionally, the presence of the double bond gives us a lot of opportunities for functionalization of our double bond into other functional groups. Due to the rigidity of the double bond, alkenes have a lot of stereochemical properties that we are going to discuss later in this course. Just like alkanes, alkenes can be both open chain and cyclic.Īs alkenes are a functional group, we are going to signify that by changing the -ane ending to an -ene ending. The important characteristic of alkenes is the presence of a carbon-carbon double bond C=C. Typical reactions of alkanes are either a simple combustion which is not very useful unless you’re trying to warm up your house or run your car, and radical halogenation. When we don’t have any other functional groups in the molecule, we use the ending -ane to signify that this is an alkane. Thus, I’m going to mention them here.Īlkanes consist of single C-C and C-H bonds, they don’t have any other heteroatoms, and they can be either open chain or cyclic. While alkanes are not technically a functional group as there’s nothing unique to them and they don’t really have much of any chemistry associated with them, they are a backbone of organic molecules. Those are the functional groups consisting of only carbons and hydrogens. We’ll start with an overview of simple hydrocarbons. So, what are the most common functional groups out there? Hydrocarbons

Importantly though, not every single part of the molecule must be some sort of a functional group. However, in addition to the aldehyde functional group we also have an alkene and an aromatic compound (arene) as well. As the name suggests, we have an aldehyde functional group in it. This is a structure of trans-cinnamic aldehyde. Thus, recognizing functional groups in a molecule will get you one step closer to identifying possible reactions and transformations you may have for that molecule. We define a functional group as a specific combination of atoms that has a unique set of chemical properties. One of those basic patterns is the recognition of the functional groups.
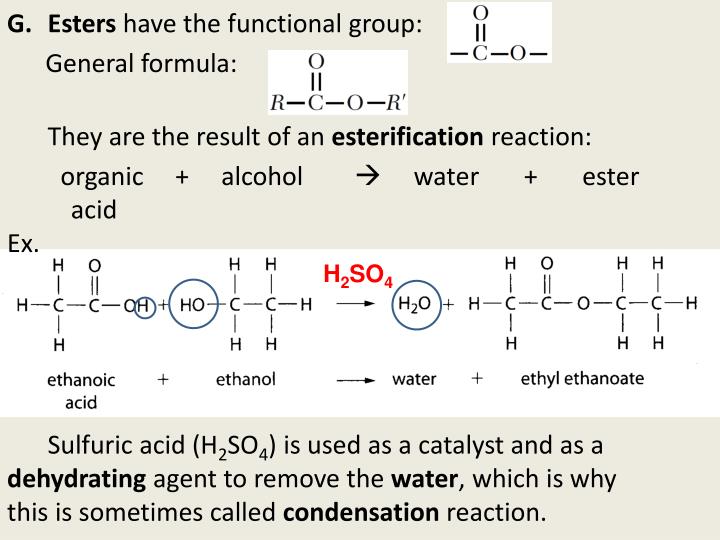
Alcohol functional group how to#
To be successful in an organic chemistry course one needs to learn how to recognize certain patterns in molecular structure and reactions.


 0 kommentar(er)
0 kommentar(er)
